Explain the Difference Between Evaporation and Sublimation
No liquid state is involved while during Vaporization liquid matter converts into Vapour. Explain the arrangement of the particles in each and give an example of each.

Give The Differences Between Evaporation And Sublimation Brainly In
What we need for evaporation is a heat source like the sun or a room with a big window that the sun can shine through or a big.
. What is the difference between evaporation and sublimation. When air temperature is decreased beyond the level of saturation. Difference Between Vaporization and Evaporation.
Hopefully you can tell the difference between the two examplesif not oh man we got to go way back to the beginning and start over. Evaporation Get the answers you need now. Evaporation is frequent when the air is dry hot and windy.
The conversion of a liquid water into a vapor a gaseous state usually through the application of heat energy during the hydrologic cycle. What is the difference between evaporation condensation and sublimation. The transition of a substance from the solid phase directly to the vapor phase or vice versa without passing through an intermediate liquid phase.
Evaporation is going from liquid to gas. It is the reversal of evaporation where liquid water becomes a vapour. What is the difference between evaporation and sublimation.
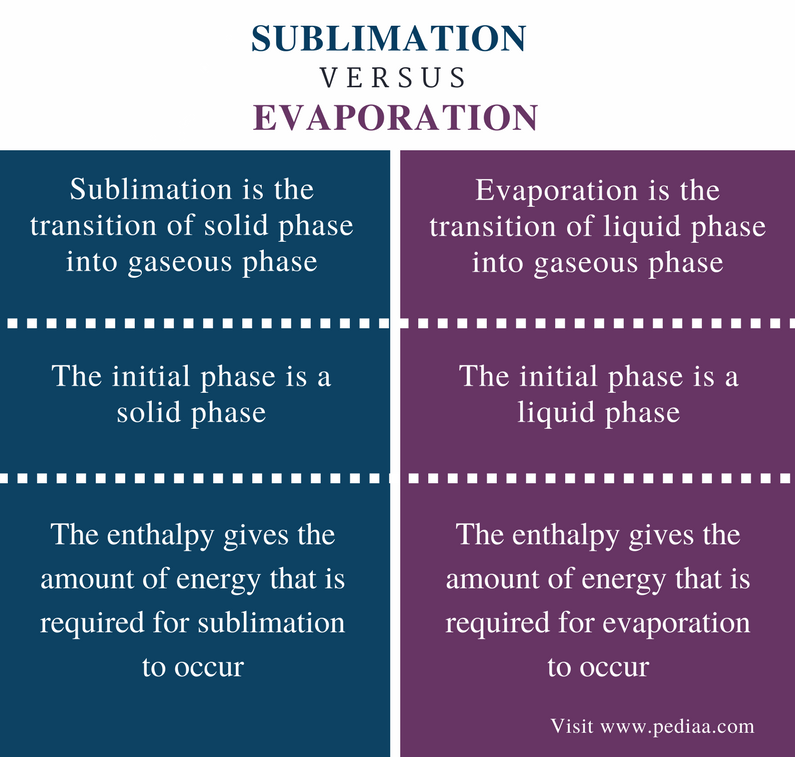
In the Sublimation process the initial phase of the matter is the solid phase while on the other hand in the. Sublimation comes from a solid changing to a gas and evaporation is liquid changing to a gas. 72 160 Review Explain the difference between sublimation and evaporation.
Sublimation is the process in which a solid goes directly into a gas. An example is a dry ice frozen carbon dioxide which does not exist in the liquid state under normal conditions. In the case of evaporation we have a.
During storms however evaporation and evapotransporation are typically not significant compared to precipitation discharge and infiltration and are often not considered. 8 rows Sublimation is the conversion of matter from a solid phase to the gaseous phase without passing. Sublimation is when something goes from the solid-state to the gaseous state without going through the liquid state.
Sublimation takes place when something goes from the solid-state to the gaseous state without undergoing the liquid state. The opposite of condensation. The main difference between Sublimation and vaporization is that during the conversion process in sublimation Solid matter turns into direct vapor.
Sublimation is simply the conversion of solid directly into the gaseous state. Explain and give one answer of each. As nouns the difference between evaporation and sublimation is that evaporation is evaporation while sublimation is.
The main difference between sublimation and evaporation is that sublimation is the phase transition from solid to gas whereas evaporation is the phase transition from liquid to gas. Sublimation is simply the conversion of solid directly into the gaseous state. Explain 5 points on difference between Sublimation amp.
Condensation takes place on salt hygroscopic nuclei-pollen grains carbon particles etc. When evaporation takes place energy is consumed. In the case of evaporation we have a.
Sudhanshupal578323 sudhanshupal578323 23022020 Chemistry Secondary School answered Explain 5 points on difference between Sublimation Evaporation 2 See answers. Explain the difference between sublimation and evaporation. Evaporation tends to lower water level in a pond or wetland over time and evapotranspiration acts to dry out the soil before the next storm.
The main difference between sublimation and evaporation is that sublimation is the conversion of matter from a solid phase to the gaseous phase whereas evaporation is the conversion of the liquid phase into the gaseous phase. So as we begin to heat the substance up or lower the pressure the solid will go directly into the gas phase without first forming a liquid. What is the difference between sublimation and condensation.
Main Differences Between Sublimation and Evaporation Sublimation is the process in which the solid- state of matter changes directly into the gaseous state of matter and. The difference between evaporation and boiling is that evaporation is the state of a liquid being transferred into a gas without boiling while boiling is a change in a liquid into a gas. -Condensation is the procedure where water vapor becomes liquid.
In the process of condensation energy is released. Sublimation is going from solid to gas. So as we begin to heat the substance up or lower the pressure the solid will go directly into the gas phase without first forming a liquid.
The water vapor a gas in the air condenses into a liquid when it gets cold enough.

Difference Between Sublimation And Evaporation With Table Ask Any Difference

Difference Between Sublimation And Deposition Compare The Difference Between Similar Terms

Difference Between Sublimation And Evaporation Definition Mechanism Examples
No comments for "Explain the Difference Between Evaporation and Sublimation"
Post a Comment